Did you undergo a hip, knee or ankle replacement?
Several Exactech implant devices have been recalled due to failure of polyethylene plastic inserts used to keep implant components from rubbing against each other. The recalled devices include Exactech Connexion GXL Hip Implant Liners, Exactech Total Knee Replacement (TKR) Optetrack, Optetrack Logic, Truliant and Exactech Total Ankle Replacement (TAK) Vantage.
Exactech Connexion GXL Hip Replacement Liners
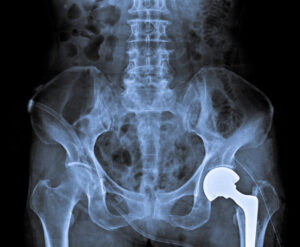 Patients who received Exactech GXL Connexion acetabular polyethylene hip replacement liners are at risk for serious injury related to device failure after a recall was issued in June 2021. The recall states premature wear and edge-loading can cause serious problems and injuries including osteolysis, limited mobility and device loosening. These types of problems may require corrective surgery.
Patients who received Exactech GXL Connexion acetabular polyethylene hip replacement liners are at risk for serious injury related to device failure after a recall was issued in June 2021. The recall states premature wear and edge-loading can cause serious problems and injuries including osteolysis, limited mobility and device loosening. These types of problems may require corrective surgery.
The FDA has received numerous adverse event reports related to Exactech Connexion GXL acetabular polyethylene liner failures. A study in Arthroplasty Today found a high rate of early device failure for many patients who received Exactech Connexion GXL acetabular liners. These early failures occurred less than five years after the initial surgery. Additionally, nine out of twelve patients examined by the study who suffered an early device failure required revision surgery to correct problems like osteolysis. Osteolysis is the destruction or loss of bone material as a result of particles from artificial ball and socket joints flaking off and impacting nearby tissues.
Free Case Evaluation
Exactech Connexion GXL Hip Liner Lawsuits
If you received an Exactech Connexion GXL hip liner and suffered bone damage, chronic joint pain after surgery, reduced mobility or device loosening contact us. We can provide you with a free case evaluation and advise you of your legal rights and options.
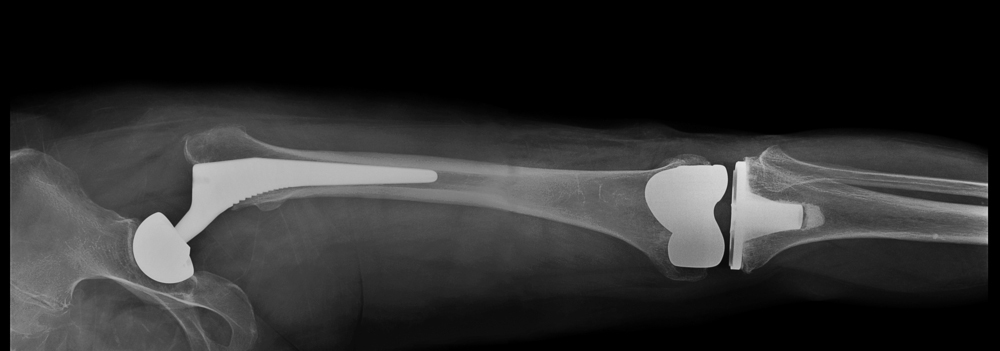
Exactech Total Knee Replacement (TKR) & Exactech Total Ankle Replacement (TAR)
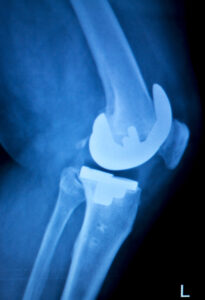 Patients who received Exactech total knee replacement or total ankle replacement implants are being notified of a product recall that can put their health at serious risk. Per the manufacturer, “most of our total knee replacements (TKR) and total ankle replacements (TAR) with polyethylene plastic components and inserts…were packaged in out-of-specification vacuum bags…”
Patients who received Exactech total knee replacement or total ankle replacement implants are being notified of a product recall that can put their health at serious risk. Per the manufacturer, “most of our total knee replacements (TKR) and total ankle replacements (TAR) with polyethylene plastic components and inserts…were packaged in out-of-specification vacuum bags…”
It was found that these out-of-specification vacuum bags did not contain a secondary barrier layer that would help with oxygen resistance of the components. This defect can lead to early device failure. Serious personal injury can occur in patients who received these defective implants including:
- Osteolysis (bone loss)
- Severe pain
- Recurrent swelling
- Reduced mobility
In addition to these injuries, patients may experience loosening of the knee or ankle. Several models of total knee replacement implants are included in this recall:
- Optetrack TKR
- Optetrack Logic
- Truliant
- Vantage (ankle replacement)
Exactech Optetrack, Optetrack Logic, Truliant, and Vantage Lawsuits
Patients have already filed lawsuits against Exactech for injuries they received by faulty Exactech knee implants. If you or someone you know had a knee or ankle replacement with an Exactech Optetrack, Optetrack Logic, Truliant, or Vantage device and suffered a serious injury, contact us. Our product liability attorneys can provide you with a free case evaluation.
CohenMalad, LLP attorneys have represented thousands of people in class action and mass tort lawsuits who were injured by large corporations including those who make dangerous drugs and medical devices. For more than 30 years we have helped injured people get compensation for their injuries. We are proud to hold these large companies accountable. Many corporations have been forced to pay back billions of dollars to the people they have injured.




